SAS programming, macro development, and migration to R or Python
Python programming support on request
Integration with R (e.g. via container platforms), SAS or Python tools
Modular setup: from assessment to architecture, deployment, validation, and knowledge transfer
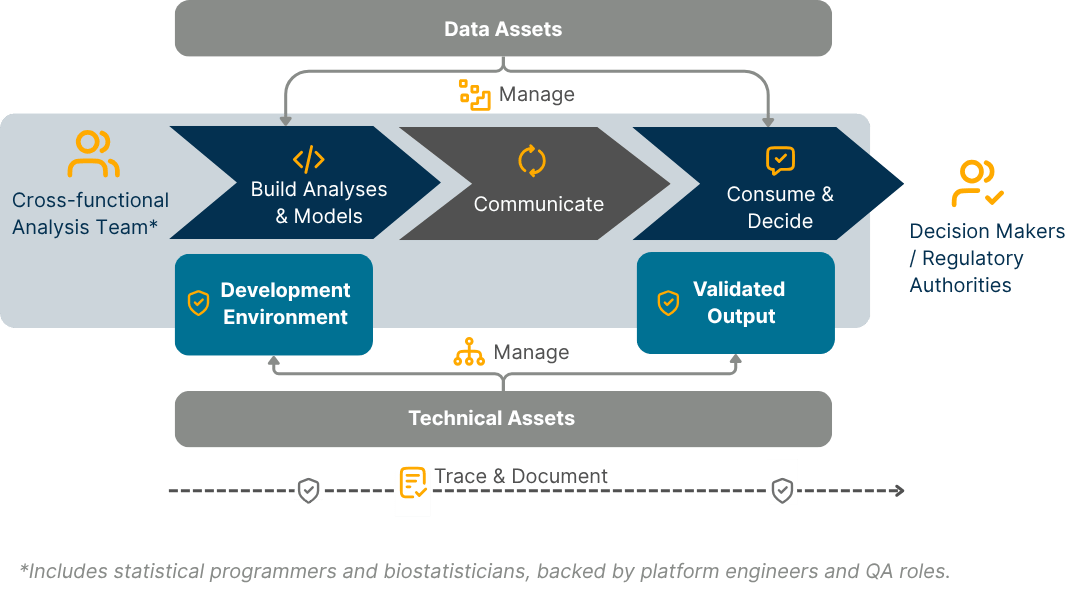
Manage data assets
Integration into SCE platforms and version control
Modernization of processes and toolchains within regulatory frameworks
Support exploratory and submission workflows
Follow IT strategy requiring platform modernization